
Sandia maintains extensive facilities for the design, synthesis, and characterization of hydrogen storage materials. Our major hydrogen storage research activities include:
- fundamental studies of hydrogen interactions with solid-state materials;
- design and synthesis of promising on-board reversible hydrogen storage materials with exothermic hydrogenation and appropriate kinetics and cycling behavior;
- understanding processing-structure-property relationships for improved materials performance through compositional, structural, catalytic, and nanostructure modification;
- developing in-situ techniques to characterize hydrogen storage materials and elucidate the role of intermediates, defects and interfaces on hydrogen diffusion and reaction pathways;
- engineering and process development to accelerate the commercial use of the best hydrogen storage materials.
Sandia’s unique capabilities are rooted in interdisciplinary research. Sandia’s interdisciplinary approach enables self-assembled materials, tailored alloys, multicomponent composites, destabilized and nanostructured metal hydrides to be conceived, synthesized, characterized and evaluated for vehicular hydrogen storage.
Multiple cutting-edge studies are carried out through collaborations with other National Laboratories, universities and companies.
HyMARC
Sandia co-leads the Hydrogen Materials Advanced Research Consortium (HyMARC). HyMARC provides the fundamental understanding of phenomena governing thermodynamics and kinetics necessary to enable the development of on-board solid‐phase hydrogen storage materials. These resources will create an entirely new capability that will enable accelerated materials development to achieve thermodynamics and kinetics required to meet Department of Energy targets.
To learn more about our hydrogen subsurface capabilities, visit Sandia’s Hydrogen Subsurface Storage webpage.
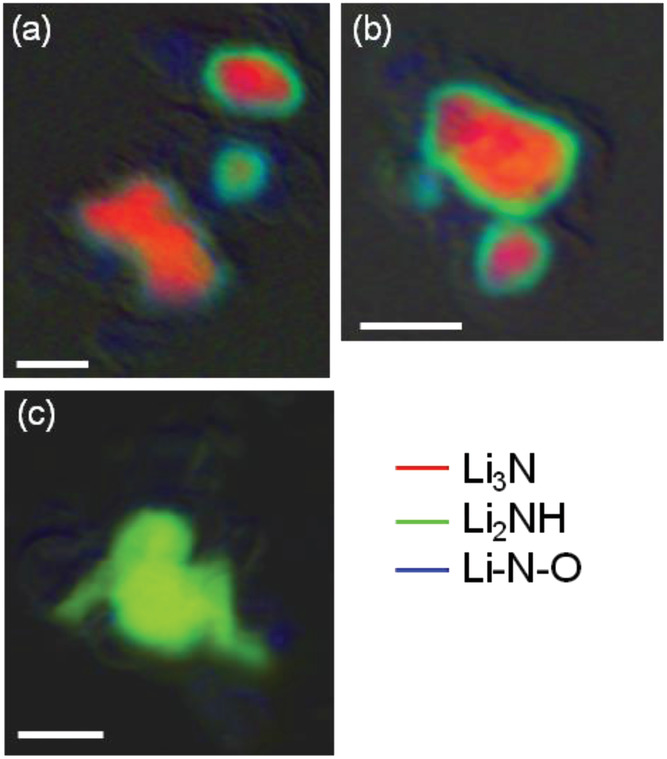
“The Inside-Outs of Metal Hydride Dehydrogenation: Imaging the Phase Evolution of the Li-N-H Hydrogen Storage System”, James L. White, Alexander A. Baker, Matthew A. Marcus, Jonathan L. Snider, Timothy C. Wang, Jonathan R. I. Lee, David A.L. Kilcoyne, Mark D. Allendorf, Vitalie Stavila, Farid El Gabaly, Advanced Materials Interfaces, 2020, 1901905.
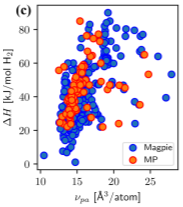
“Extracting an Empirical Intermetallic Hydride Design Principle from Limited Data via Interpretable Machine Learning”, Matthew Witman, Sanliang Ling, David M. Grant, Gavin S. Walker, Sapan Agarwal, Vitalie Stavila, and Mark D. Allendorf, Journal of Physical Chemistry Letters, 2020, 11, 40-47.

“Identifying the Role of Dynamic Surface Hydroxides in the Dehydrogenation of Ti-Doped NaAlH4”, J.L. White, A.J.E. Rowberg, L.W.F. Wan, S. Kang, T. Ogitsu, R.D. Kolasinski, J.A. Whaley, A.A. Baker, J.R.I. Lee, Y.-S. Liu, L. Trotochaud, J.G. Guo, V. Stavila, D. Prendergast, H. Bluhm, M.D. Allendorf, B.C. Wood, F. El Gabaly, ACS Applied Materials and Interfaces, 2019, 11, 4930-4941.
Contact
Mark Allendorf
(925) 294-2895
mdallen@sandia.gov